By Harshit
CAMPINAS, BRAZIL — DECEMBER 7, 2025
A hormone produced in the intestine may play a powerful role in how the brain controls energy use, fat burning, and metabolic health, according to new research that sheds light on potential future treatments for obesity and diabetes.
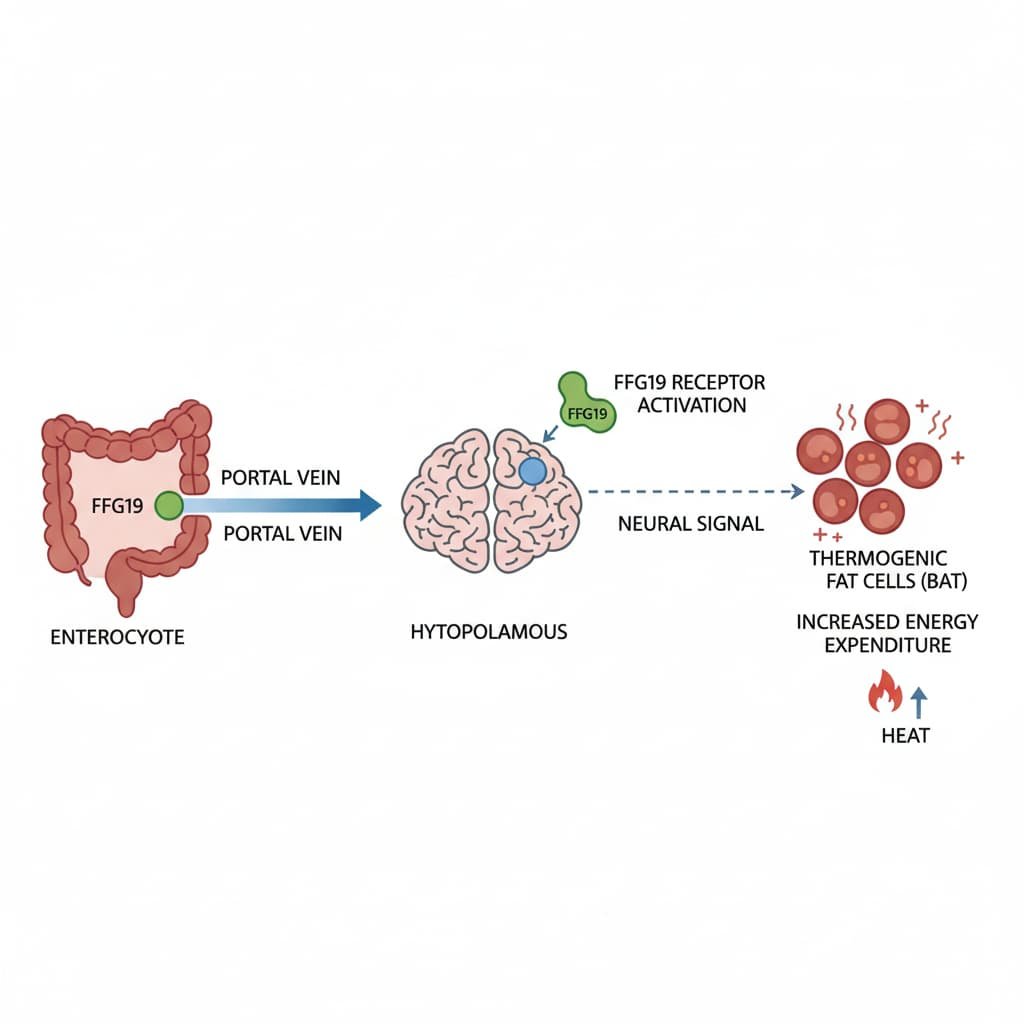
Scientists studying obese mice have found that fibroblast growth factor 19 (FGF19), a hormone released by the small intestine, sends signals directly to the hypothalamus — a critical brain region involved in regulating hunger, body temperature, and metabolism. Once activated, this brain pathway increases energy expenditure, stimulates fat-burning cells, and improves glucose control, even without changes in food intake.
The findings suggest that the gut–brain connection driven by FGF19 could be harnessed to develop new metabolic therapies that go beyond appetite suppression and instead help the body burn excess energy more efficiently.
How the Brain Controls Fat Burning
The hypothalamus acts as a central command center, continuously receiving information from organs such as the gut, liver, and fat tissue. It then issues signals that influence how much energy the body stores versus how much it burns.
In this study, researchers discovered that FGF19 activates specific hypothalamic circuits that increase the activity of thermogenic adipocytes — specialized fat cells that burn calories to generate heat rather than storing them. These include brown fat and “beige” fat cells embedded within white fat tissue.
When these cells are switched on, the body’s overall energy expenditure rises, helping counteract obesity-related metabolic dysfunction.
“Our work shows that FGF19 does more than regulate appetite,” said Professor Helena Cristina de Lima Barbosa of the Obesity and Comorbidities Research Center (OCRC) at the State University of Campinas (UNICAMP). “It also acts on the hypothalamus to stimulate thermogenesis. From a therapeutic perspective, that makes a great deal of sense for obesity.”
Beyond Appetite: Energy Expenditure Takes Center Stage
Unlike many current weight-loss strategies that focus primarily on reducing food intake, FGF19 appears to influence metabolism directly. In the experiments, obese mice receiving FGF19 signaling in the brain showed:
- Increased energy expenditure
- Activation of fat-burning thermogenic tissue
- Improved blood glucose control
- Reduced peripheral inflammation
- Better tolerance to cold exposure
Notably, these effects disappeared when researchers blocked the sympathetic nervous system — the network responsible for regulating involuntary functions such as heart rate and heat production. This finding confirms that FGF19’s metabolic benefits depend on brain-to-body neural signaling.
Further experiments showed that exposure to cold increased the expression of FGF19 receptors in the hypothalamus, reinforcing the hormone’s role in coordinating energy balance and temperature regulation.
Parallels With Modern Obesity Drugs
The approach resembles how some of today’s most effective metabolic drugs work. For example, semaglutide — the active ingredient in medications such as Ozempic — mimics the hormone GLP-1 to send satiety signals to the brain.
FGF19 follows a similar biological philosophy: rather than forcing artificial pathways, it amplifies natural hormonal signaling already present in the body.
“The idea is to mimic endogenous compounds — substances the body already produces — and use them more effectively,” the researchers noted.
Where FGF19 Comes From
FGF19 is primarily released by the small intestine after meals, under regulation by bile acids. In the liver, it is known to control bile acid production and influence glucose and lipid metabolism. However, its direct effects on the brain have remained relatively unexplored.
Graduate researcher Lucas Zangerolamo, the study’s first author, said the team’s interest in bile acids helped lead them to uncover FGF19’s central role in brain-based metabolic regulation.
How the Study Was Done
At eight weeks of age, mice were divided into two groups: one fed a standard diet and the other fed a high-fat diet to induce obesity. The obese animals then received FGF19 directly into the brain under carefully controlled conditions.
The researchers also analyzed large public datasets using single-cell RNA sequencing to identify which hypothalamic cells express receptors for FGF19. They examined gene activity from more than 50,000 individual brain cells, allowing them to pinpoint specific cell populations involved in the hormone’s effects.
The results showed that central FGF19 signaling enhances energy homeostasis by activating sympathetic pathways that drive thermogenesis in adipose tissue.
A Growing Global Health Challenge
The findings arrive amid a worsening global obesity crisis. According to the World Atlas of Obesity 2025, more than one billion people worldwide are currently living with obesity, a figure projected to exceed 1.5 billion by 2030 if trends continue.
Obesity is already linked to approximately 1.6 million premature deaths each year due to non-communicable diseases such as cardiovascular illness, diabetes, and cancer.
In Brazil, about 31% of the population has obesity, and nearly half of adults do not meet recommended physical activity levels.
What Comes Next
While the research was conducted in mice, the biological pathways involved are conserved across mammals, raising optimism about future human applications. The next challenge is determining how to safely stimulate FGF19 pathways without direct brain injections.
“We want to understand whether it’s possible to boost natural FGF19 production or design compounds that selectively activate its brain effects,” Zangerolamo said.
The team is also investigating whether FGF19 can reduce hypothalamic inflammation associated with high-fat diets — a known driver of obesity-related metabolic dysfunction.
The study was published in the American Journal of Physiology – Endocrinology and Metabolism and was highlighted as a Top Article in May. Funding was provided by FAPESP through the Obesity and Comorbidities Research Center (OCRC).
As scientists continue exploring the gut–brain axis, FGF19 stands out as a promising signal — one that could shift obesity treatment from simply eating less to burning more.