By Harshit
College Station, Texas | November 12, 2025 | 04:20 AM EDT
In a breakthrough that could redefine the future of cancer therapy, scientists at Texas A&M University have successfully produced and distributed Astatine-211 (At-211) — one of the world’s rarest and most unstable elements — for use in targeted cancer treatment. Despite its fleeting existence and mere 7.2-hour half-life, this elusive isotope is earning the nickname the “Goldilocks” element for its perfect balance of power and precision in destroying cancer cells while sparing healthy tissue.
A Rare Element Finds Its Purpose
Astatine is so scarce that, at any given time, less than a gram exists naturally on Earth. Its name, derived from the Greek astatos (meaning “unstable”), reflects its fleeting presence — but within that instability lies immense therapeutic potential.
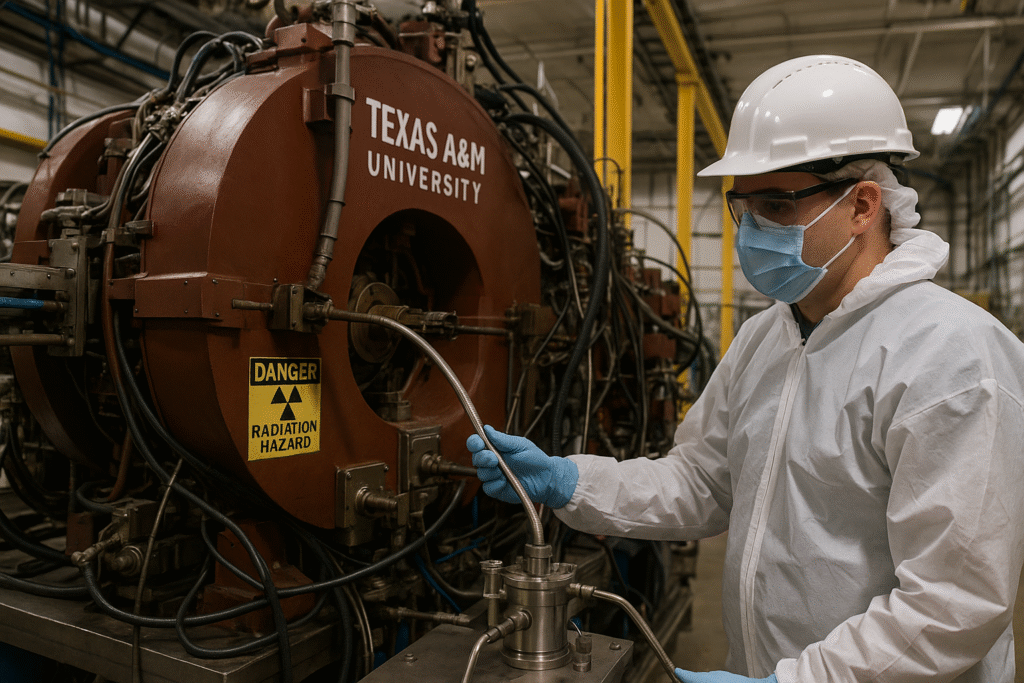
Using the K150 cyclotron at Texas A&M’s Cyclotron Institute, researchers have developed an advanced process to produce, isolate, and purify At-211 in medically viable quantities. The work is being conducted in collaboration with the U.S. Department of Energy (DOE) Isotope Program, which funds national isotope production for scientific and medical applications.
“Targeted alpha therapy is a potentially transformative cancer therapeutic,” said Dr. Sherry J. Yennello, Distinguished Professor of Chemistry and Director of the Cyclotron Institute. “It can cause extensive damage to cancer cells while keeping healthy tissue intact. We’re proud that Texas A&M is one of the few centers capable of routinely producing astatine for this purpose.”
Since 2023, Texas A&M has become one of only two national suppliers of At-211 through the DOE’s National Isotope Development Center (NIDC) and its University Isotope Network.
The ‘Goldilocks’ of Isotopes
What makes At-211 so special is its alpha radiation — short-ranged but highly energetic emissions composed of two protons and two neutrons. These alpha particles deposit their energy over a distance of just a few cell diameters, making them devastatingly effective at killing tumor cells while minimizing collateral damage.
Unlike traditional beta-emitting isotopes, which can penetrate deeper and harm nearby organs, At-211’s short travel distance ensures surgical precision.
“Astatine’s radiation is just right — powerful enough to destroy cancer cells but localized enough to avoid damaging surrounding tissues,” said Yennello. “That’s why we call it the ‘Goldilocks’ isotope.”
The isotope’s short half-life also makes it safer than many existing radiopharmaceuticals. Within hours of treatment, most of the radioactive material decays naturally, leaving behind minimal residual radiation.
From Cyclotron to Clinic: The Texas A&M Advantage
One of the team’s most significant achievements is the development of a patent-pending automated system for isolating and transporting At-211. Traditionally, separating the isotope from its bismuth target was a slow and hazardous process, resulting in significant decay before clinical use.
Texas A&M’s resin-column trapping method solves this problem by speeding up purification and packaging. Once isolated, the isotope is attached to a special shipping column that enables safe, quick transport to medical research facilities — often within hours of production.
This innovation has allowed the university to supply At-211 to multiple partners, including:
- MD Anderson Cancer Center, which has received more than two dozen shipments,
- University of Alabama at Birmingham, and
- UTHealth Houston, where researchers are investigating new radiopharmaceutical formulations.
“Availability has always been the bottleneck,” Yennello noted. “With our improved production and shipping process, we’re helping bridge the gap between laboratory science and clinical medicine.”
Harnessing Alpha Power for Precision Therapy
When delivered directly to a tumor via targeted alpha therapy, At-211 acts like a microscopic guided missile. Researchers attach the isotope to monoclonal antibodies — molecules engineered to bind specifically to cancer cells. Once the antibody finds its target, the alpha radiation from At-211 destroys the malignant cell from within, while nearby healthy cells remain largely unharmed.
Clinical trials are currently exploring At-211’s potential for treating:
- Blood cancers such as leukemia,
- Ovarian and thyroid tumors, and
- Certain brain cancers, where its short-range radiation is especially valuable.
Early studies have also hinted at potential applications in neurodegenerative diseases like Alzheimer’s, where precisely controlled radiation could disrupt harmful protein accumulations without damaging healthy neurons.
A Growing Global Collaboration
Texas A&M’s pioneering work has made it a global leader in At-211 production and research. Yennello and her collaborator, Dr. Federica Pisaneschi, a former MD Anderson radiochemist now at UTHealth Houston, will present their latest findings at the upcoming 2025 World Astatine Community Meeting in New Orleans. Their talk — titled “The Texas Two-Step” — will detail the team’s dual progress in isotope production and clinical application.
Yennello also recently addressed the 26th International Symposium on Radiopharmaceutical Sciences in Queensland, Australia, where she emphasized rising international interest in At-211.
“We’re seeing coordinated efforts in Japan, across Europe, and here in the U.S. to explore astatine’s chemistry and therapeutic potential,” she said. “It’s a very exciting time for nuclear medicine.”
The Road Ahead: Expanding Astatine’s Reach
The Texas A&M team continues to refine its techniques for scaling production, extending isotope lifespan during transport, and improving tumor-targeting precision.
Supported by the DOE Office of Science, the Bright Chair in Nuclear Science, and the Texas A&M University System Nuclear Security Office (in partnership with Los Alamos National Laboratory), this initiative could soon lead to broader clinical access for patients nationwide.
If ongoing trials confirm At-211’s safety and effectiveness, it could emerge as the next generation of radiotherapeutic medicine — one that provides surgical precision at the molecular level without the side effects of traditional radiation therapy.
From Rarity to Remedy
Once dismissed as too unstable to be useful, astatine is finally proving its worth. Its fleeting nature — a challenge for scientists for decades — may now be its greatest strength, offering a controlled and finite burst of healing radiation where it’s needed most.
“We’re turning one of nature’s rarest and most unstable elements into a tool for healing,” said Yennello. “That’s the beauty of science — finding balance between instability and possibility.”