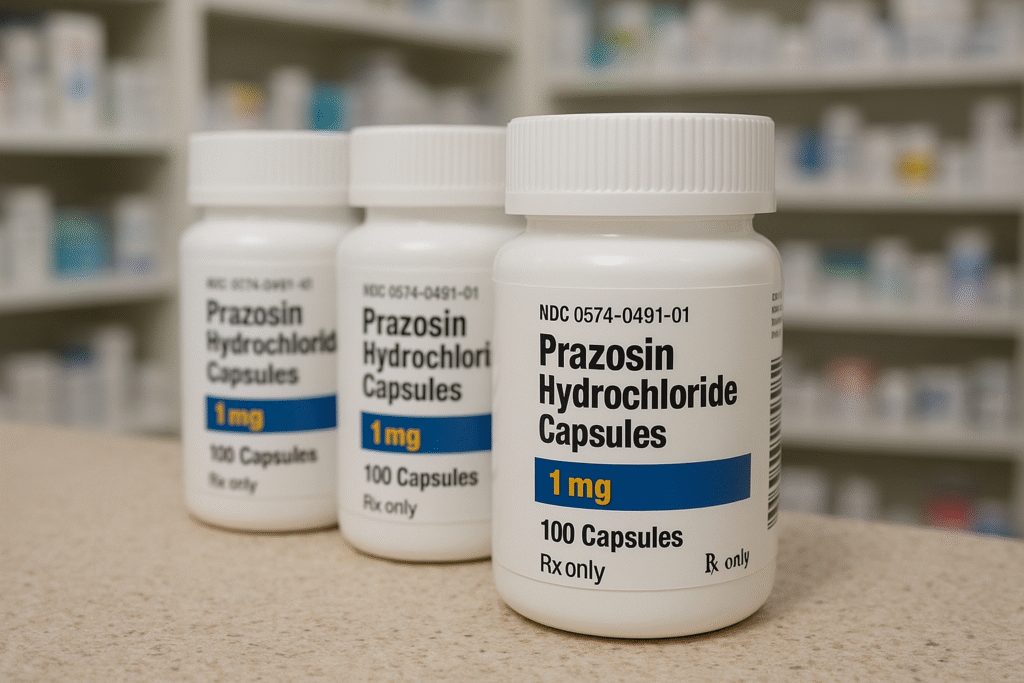By Harshit, NEW YORK, Oct. 31, 2025
In a significant pharmaceutical recall, Teva Pharmaceuticals USA Inc. has voluntarily withdrawn more than 580,000 bottles of a widely used blood pressure medication after federal testing found traces of a potentially cancer-causing compound.
The U.S. Food and Drug Administration (FDA) confirmed in an enforcement report that Teva, based in New Jersey, initiated the recall of Prazosin Hydrochloride capsules — sold in 1 mg, 2 mg, and 5 mg doses — after identifying “above acceptable intake limits” for N-nitroso Prazosin impurity C, a chemical linked to cancer risks.
Details of the Recall
According to FDA documents, the recall began on October 7 and was later classified as a Class II recall on October 24, indicating that while the product “may cause temporary or medically reversible adverse health consequences,” the chance of serious harm is considered low.
A memo from the California Board of Pharmacy stated that Teva’s internal health hazard assessment deemed the potential harm to patients as “medium.” The recall affects specific lots of the medication that were distributed nationwide.
The FDA advises consumers to check the lot codes of any Prazosin bottles in their possession to determine if they are affected. A full list of impacted lots has been published by Teva Pharmaceuticals and is available through the FDA’s recall database.
What Is Prazosin?
Prazosin, also sold under the brand name Minipress, is an alpha-blocker prescribed to manage hypertension (high blood pressure). It works by relaxing blood vessels so blood can flow more easily, helping reduce cardiovascular strain.
The drug is also sometimes prescribed off-label for conditions such as post-traumatic stress disorder (PTSD)-related nightmares and certain cases of benign prostatic hyperplasia (BPH).
Public Health Concerns
Although Teva has stated that the likelihood of severe health effects is remote, experts caution that nitrosamine impurities — like the one detected in this recall — have been linked to cancer risk over long-term exposure.
The FDA has been actively investigating nitrosamine contamination in several medications over the past few years, particularly in blood pressure, diabetes, and heart medications. In most cases, manufacturers have voluntarily recalled affected batches to prevent potential exposure.
Teva’s Response
Teva Pharmaceuticals has yet to issue a formal public statement about the Prazosin recall. However, the company’s past actions in similar situations suggest it will continue to cooperate with FDA safety protocols and testing procedures.
Consumers who are currently taking Prazosin are advised not to stop using the medication abruptly without consulting a healthcare professional, as doing so could cause blood pressure spikes and other complications. Patients can contact Teva or the FDA for guidance on replacement options.
Recent Recall Trend
This is not the first major recall of a cardiovascular drug in recent months. In September, a recall was issued for Lipitor, a cholesterol-lowering medication, due to “failed dissolution specifications” — meaning the pills did not dissolve properly, potentially reducing their effectiveness.
The wave of recalls highlights growing industry challenges in maintaining manufacturing consistency and chemical purity across complex supply chains.
Looking Ahead
The FDA continues to emphasize that while the presence of nitrosamine impurities poses a concern, patients should not panic or discontinue medication without medical advice. Regulatory experts stress that recalls such as these demonstrate the strength of the U.S. drug monitoring system rather than its failure.
Healthcare professionals are now urging patients to verify their medication lot numbers and report any adverse reactions directly to the FDA’s MedWatch program.
As the recall investigation progresses, more information is expected on how the contamination occurred and what preventive steps will be taken by Teva to ensure future product safety.